Multicolored vertical silicon nanowires
Bulk silicon appears gray in color, though it can be made black if its reflectance is reduced by nanostructuring. Vertical silicon nanowires, however, take on a surprising variety of colors under bright-field illumination covering the entire visible spectrum. The reflection spectra each exhibit a dip, the position of which redshifts as the nanowire radius is increased, enabling fine control of the observed color. For experimental convenience, we fabricate nanowires in arrays. The spectral dip does not however arise from scattering or diffractive effects of the array, but from the waveguide mode properties of the individual nanowires. Each nanowire can thus define its own color independent of its neighbors, allowing for complex spatial patterning. This surprising phenomenon may lead to greater sensitivity in image sensor devices.

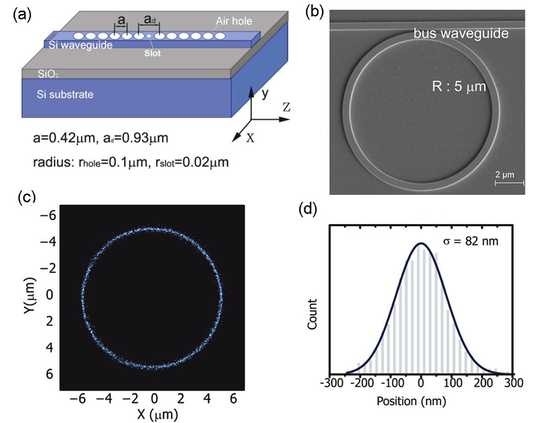
Patterned vertical silicon nanowire arrays. (a) 30º tilted SEM image of vertical silicon nanowire array pattern. Nanowires are ~1 µm long and have a pitch of ~1 µm. Letters S (left), E, A, and S (right) each comprise nanowires with radii of 70 nm, 60 nm, 50 nm and 40 nm, respectively. Bars above and below letters consist of nanowires with radii varying from 75 to 35 nm (left to right). (b) Bright-field optical microscope image of pattern. (c) Magnified image of area indicated by the white square of panel b. Each blue spot is a single nanowire. (d) 30º tilted SEM image of Bayer filter pattern. Pattern consists of vertical silicon nanowires with radii of 45 nm, 50 nm, and 65 nm representing red, blue, and green colors, respectively. Inset: magnified SEM image. Scale bar is 1 µm. (e) Bright-field optical microscope image of pattern. Each nanowire shows a color that can be controlled by appropriate choice of its radius.
This work could find applications in color image sensors based on nanowires. Each pixel would consist of a nanowire (acting as a photodetector) formed above a second photodetector in the substrate. Part of the spectrum would be detected by the nanowire photodetector, and part by the substrate photodetector. In this way, one could perform color separations in an efficient manner.
Reference:
“Multicolored Vertical Silicon Nanowires,” Kwanyong Seo, Munib Wober, Paul Steinvurzel, Ethan Schonbrun, Yaping Dan, Tal Ellenbogen, and Kenneth B. Crozier, Nano Letters vol. 11, pp 1851–1856 (2011)
Surface passivation of nanowires
Our group is interested in developing solar cells and photodetectors based on nanowires and microwires. Due to their large surface-to-volume ratios, however, nanowires have a high surface recombination rate. This can shorten the carrier lifetime by 4-5 orders of magnitude, making optoelectronic devices based on nanowires have low efficiency. Methods for the surface passivation of planar optoelectronics are well developed. The surface passivation of nanowires, however, is more challenging, due to their small size and the fact that multiple facets (with different crystalline orientations) are exposed. We have recently developed a highly effective means for surface passivation of silicon nanowires, using a thin layer of amorphous silicon formed in situ during nanowire growth (NanoLetters vol. 11, 2527 (2011)).
Silicon nanowires are synthesized by the vapor-liquid-solid (VLS) process. The amorphous surface passivation layer is formed during nanowire growth. The mechanism by which the core-shell structure (Figure 1a) is formed is still under investigation.
To characterize the surface recombination rate, we perform scanning photocurrent microscopy (Figure 1b). After making electrical contacts, the nanowire is mounted in a near-field scanning optical microscope. This enables the photocurrent, produced in response to the illumination from the aperture near-field probe, to be found as a function of probe position (Figure 1b bottom right). The topography is found at the same time (Figure 1b bottom left). From the photocurrent maps, the diffusion lengths are found for nanowires with different diameters (Figure 1c). Measurements are made both on core-shell nanowires, and on nanowires without shells. From the data, we extract the surface recombination rate. The results demonstrate that the surface recombination rate is reduced by about two orders of magnitude using the amorphous silicon coating. We further explore this phenomenon by comparing the photosensitivity of core-shell nanowires and nanowires without shells with similar diameters. These measurements are performed with light from a spectrometer, i.e. the photosensitivity is found as a function of wavelength. We find that the core-shell nanowires have photosensitivities about two orders of magnitude stronger than those of the nanowires without shells.

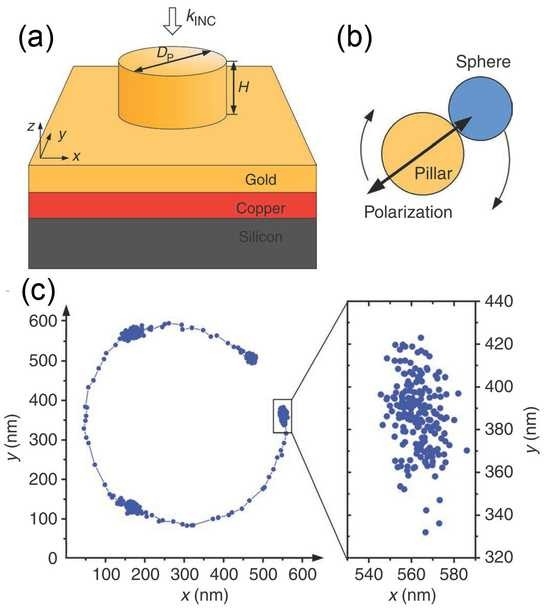
(a) TEM image, showing that amorphous shell is ∼10 nm thick and crystalline core is ∼40 nm in diameter. Inset: energy dispersive X-ray spectroscopy (EDS) indicates that the amorphous shell is silicon. Scale bar, 30 nm. (b) Top: conceptual illustration of method for measuring carrier diffusion lengths. Bottom left: nanowire topography, obtained by AFM functionality of near-field scanning optical microscope. Bottom right: Photocurrent map, recorded simultaneously with topographic image. (c) Surface passivation effects in nanowires of different diameters. Diffusion length vs nanowire diameter, for core-shell (black squares) and regular nanowires without shell (red dots and green stars).