
Breaking Bad bugs: Testing potential biocontrol agents for pesticide tolerance
- Words by Ashritha Dorai
- Banner art by Lucy Hayward
I have a passion for playing mad scientist with pesticides, all in the name of making agricultural pest control safer and more sustainable.
If you’re fond of growing roses, you’re probably familiar with the frustrating critters that invade them in your garden. The critters, in this case aphids, are unfortunately fond of your roses, along with over 40 different plant families including plums, peaches and peonies. They cause damage to plants by feeding on sap and transmitting plant viruses.

Damage to plant health via virus transmission (credit: Joop van Leur)
Current methods of controlling aphids largely rely on the use of chemical pesticides, where overuse has led to resistant populations, limiting the effectiveness of the pesticides. Pesticides also have a significant adverse impact on ecosystems and biodiversity. Aphids reproduce in large numbers quickly, due to their ability to asexually clone themselves, so a sustainable method of control is imperative.
Research from our lab has revealed that certain endosymbiontic bacteria that have co-evolved inside the cells of aphids, can be manipulated to alter aphid health and become a potential biocontrol agent. The endosymbiont Rickettsiella viridis was obtained from a donor aphid species called the Pea aphid, and transferred into a target aphid species, the green peach aphid (herein, “GPA”), via microinjection. Remarkable results show that infected GPA have reduced heat tolerance, reduced offspring and have a change in body colour
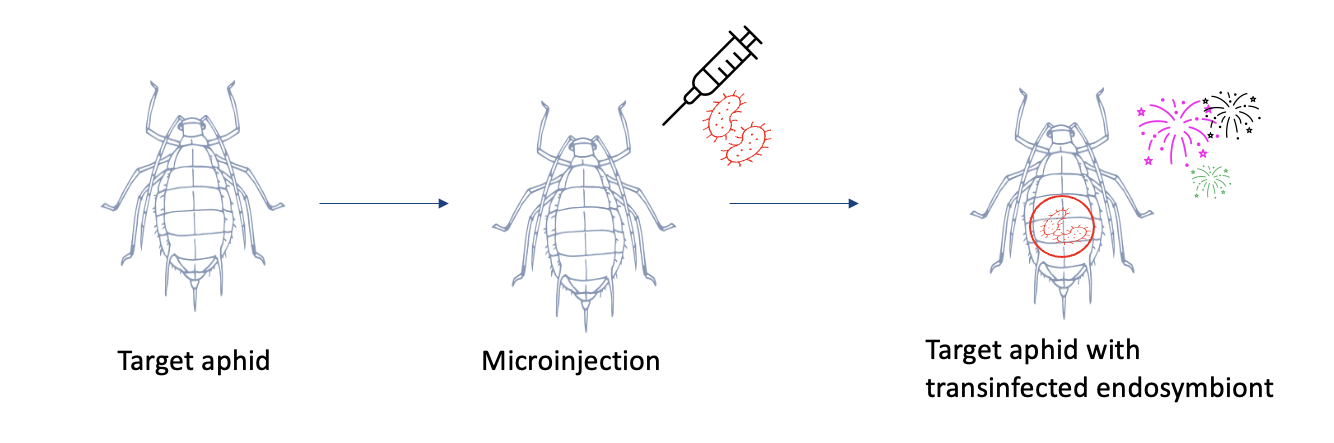
Manipulation of bacterial endosymbionts via microinjection to transinfect target aphid species.
Whilst reducing offspring numbers is desirable for biocontrol, there is a gap in knowledge about how the transinfection may affect pesticide tolerance. It is very important that novel sustainable strategies are compatible with traditional chemical control methods, as an aphid with increased tolerance is harder to kill, resulting in more chemicals being used, and in turn potentially more negative environmental impacts.
My project focuses on testing pesticide tolerance of aphid species after Rickettsiella transinfection. The PEARG lab has established successful transinfections in three aphid species; GPA, the oat aphid (herein, “OAT”) and the Russian wheat aphid (herein, “RWA”), but the endosymbionts effect on pesticide tolerance had never been tested. To test pesticide tolerance, first, leaf discs are cut and dipped in increasing pesticide concentrations and left to dry for a few hours. As these are “contact” pesticides, they rely on the insect touching the pesticide in order to be effective. Aphids are carefully placed on the leaf discs which are in petri dishes with agar gel to keep the leaves fresh. Aphids are scored after 72 h, and the results compare survival in the Rickettsiella infected line with the uninfected line.

Chemical bioassays set up with leaf discs and aphids.
The experiments showed that the Rickettsiella transinfection has no effect on pesticide tolerance in two important classes of chemicals; synthetic pyrethroids and carbamates. For all combinations of pesticide and aphid species, there was no statistical support for the Rickettsiella transinfection affecting tolerance. The overlapping concentration-response curves for each strain of GPA, OAT and RWA for Alpha-cypermethrin (Figure 1a), Bifenthrin (Figure 1b) and Pirimicarb (Figure 1c). This work suggests that there is no clear interspecific variation of Rickettsiella transinfection on pesticide tolerance.
Concentration-response curves for three aphid species GPA, OAT and RWA for Alpha-cypermethrin, Bifenthrin and Pirimicarb. The x-axis shows the pesticide concentration (mg/L), and the y-axis is the mortality (%). Small points (circle, triangle, aConcentration-response curves for three aphid species GPA, OAT and RWA for Alpha-cypermethrin, Bifenthrin and Pirimicarb. “RCL” indicates Rickettsiella transinfection. The x-axis shows the pesticide concentration (mg/L), and the y-axis is the mortality (%). Small points (circle, triangle, and square) represent means at each concentration per run and large crosses represent means across runs. Colours indicate strain, see legend.
The results are promising for the potential use of Rickettsiella as a biocontrol agent, since pesticide tolerance in one clone of three aphid species are not affected. However, one species can have many types of clones (genotypic variations) with different tolerances. Therefore, it is important to test different clones of the same species. The second chapter of my project tests further clones of green peach aphid against four classes of pesticides and an important other class of control, biological pesticides such as the fungus Beauvaria bassiana.
For those interested in delving deeper into the realm of biocontrol agents and the intricate dynamics of pesticide tolerance in aphids, I welcome you to connect with me via email, to explore this captivating topic further. I look forward to engaging in enlightening conversations with fellow enthusiasts and experts alike. aprithivsiva@student.unimelb.edu.au